

Differentiated Antibodies
Multispecifics Design
Trican Biotechnology focuses on the construct and development of innovative therapeutic modalities that address unmet medical needs.
History
Trican Biotechnology, founded in 2015, is designing antibodies based on in vitro and in silico tools for in vivo validation. Through open technology integration with our proprietary platform, Trican delivers differentiated antibodies to address disease setting across oncology, immunology, and ophthalmology.
We are actively partnering globally with antibody-drug conjugates (ADCs), drug delivery, and bispecific/multispecific innovators.

Vision
Innovation, Integrity, Compassion
Guided by the beliefs for innovation, integrity, and compassion for all humanity, Trican Biotechnology strives to continuously advance our antibody engineering platform for speed and quality. We are singularly focused on driving forward novel antibody-based innovations to clinical studies. No one company can do everything. Thus, we aim to create candidates in collaboration that address unmet medical needs, improve the quality of life for patients, and create value for all stakeholders in the innovation ecosystem.
Technologies
Briefly, Trican Biotechnology harvested and assembled tumor reactive B cell receptors sequences into our proprietary DART library ''Directed and Activated immune Response against Tumors/lesions''.
Next-gen sequencing revealed more than 200 billion binders from DART with fully-human immunogenetics after recombination. For discovery campaigns Trican applies our proprietary high-throughput selection SERA ''Serially Enriched Recombinant Antibodies'' methodology.
Binders or SERAbodies from DART library by Trican are selected based on diversity, specificity, developability, and finally affinity against targets of interest. Trican engineers can further format and characterize SERAbodies into IgG or multispecifics candidates with targeted functionalities for further clinical development.
Multiple patents have been granted based on SERAbodies raised by Trican.
SERAbody Platform
''Serially Enriched Recombinant Antibodies'' Platform
DART
''Directed Activated immune Response against Tumors/lesions'' B-Cell Receptor Library

SERA
''Serially Enriched Recombinant Antibodies'' High-Throughput Selection platform

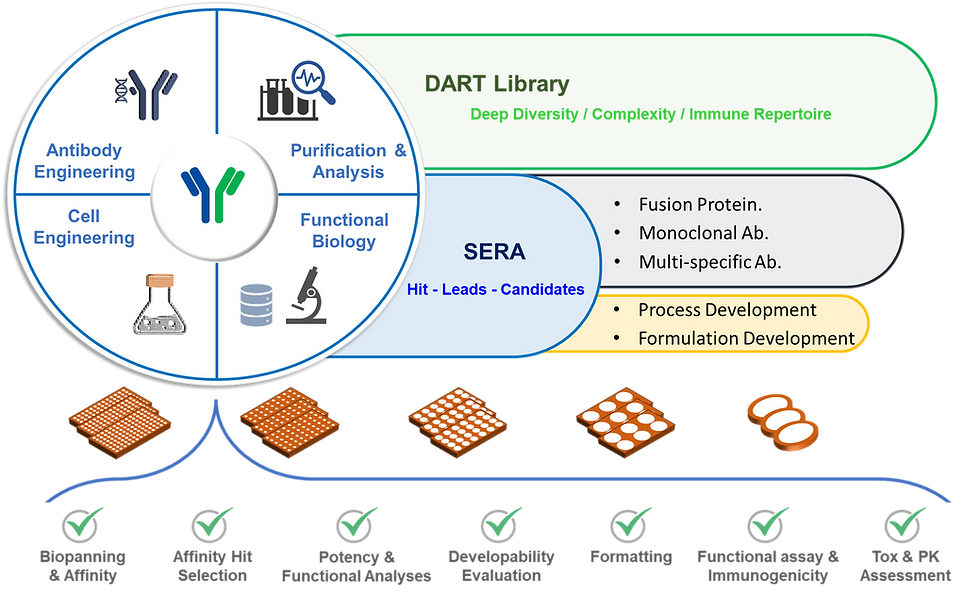
Trican's Antibody Discovery Mapping
SERAbody Portfolio
Project ID
Construct
Indication
Status
TRB007
bispecific
Ophthalmology
Optioned
TRB817
bispecific
Ophthalmology
Optioned
TRB303
IgG
Oncology
Partnered
TRB826
IgG
Oncology
Partnered
TRB824
Bispecific
Oncology
In development
TRB301
IgG
Nephrology
Ready
News
Press Release, Updates, Announments
Contact
Head Office
24F-7 No.99 Section1, Xintaiwu Road,
Xizhi District, New Taipei City, 221
Inquiries
For any inquiries, questions or commendations, please call: +886-2-2697-2685 or fill out the following form
Employment
To apply for a job with Trican Biotech,
please see detailed information on the 104 website: www.104.com.tw
